Medical Device, Fluid Bag and Flexible Packaging Design & Development Services

Development
Our product and development engineers create solutions that take customers’ ideas from the drawing board to prototype to completion as quickly and cost-efficiently as possible.
Vonco utilizes a proven, scalable and standard methodology for all projects. Success is achieved through strong project management, individualized attention, customer collaboration and a commitment to achieving key objectives including on-budget and on-time delivery.
Full-Service Engineering and Development
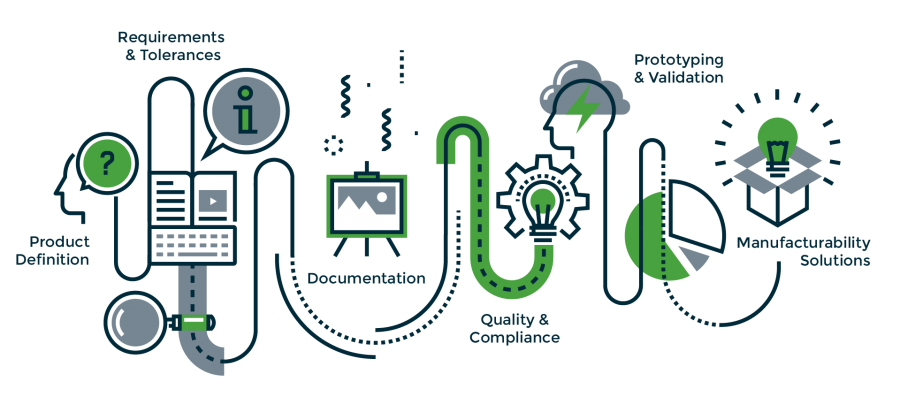
The Vonco product engineering and development continuum includes:
- CAD Design: We create tooling and product drawings that can be electronically transmitted to our customers for review.
- Prototype Tooling: Soft prototype tooling allows inexpensive validation of product design and materials prior to investing in production tools.
- Material Selection Guidance: Vonco has access to an extensive database of raw materials, as well as a wide range of polymers in-house for quick and efficient product development and testing.
- Rapid Prototyping: We can create cost-effective functional models in about two weeks from design approval – a highly accelerated turnaround time compared to the 6 to 10-week industry standard.
- Weld Testing: Vonco will weld test polymers furnished by the customer and provide a comprehensive report of product performance.
- Supplier Collaboration: Vonco has developed strong relationships with many of the industry’s leading resin and film suppliers. These collaborative relationships help facilitate our own position as a market leader as we work together to evaluate heat sealing solutions with the newest and most cutting-edge materials.

Expert guidance from initial concept to FDA clearance
Midwest-based Vonco, an outsourced medical device design and development services provider, strategically overcomes design hurdles and guides clients from initial concept to FDA clearance and commercialization, through:
- 30+ years of medical device manufacturing expertise
- Industry collaboration
- Cutting edge innovation
- Material and component expertise
- Clear documentation and processes
- Risk mitigation
- A robust QMS, as well as ISO 13485 certification


Evolving MedTech Design and Engineering for Launch Success
Quick Quote
Use this quick form to request more information from one of our experienced product experts.
Already know what you need? Click here
"*" indicates required fields

