
Image Credit: Vonco Products
It’s still early in 2024, but the globe is already marked by unprecedented events: wars in Ukraine and Israel, commercial transport bombings in the Red Sea, stubborn inflation, contentious elections, and the real possibility of another pandemic. Now more than ever, the resilience of supply chains is of paramount concern for businesses across industries, none more so than the medical device sector.
According to Medical Product Outsourcing, “rising labor costs and supply chain disruptions are causing medical device manufacturers to reevaluate their overseas outsourcing strategies.” Because the cost of manufacturing remains a priority, OEMs are building more resilient supply chains and mitigating geo-political risks by moving sourcing and production closer to consumers.
Unlike other industries, Medtech faces unique challenges given the critical nature of its products on healthcare. As we saw during the COVID-19 pandemic, disruptions in supply chain can have a devastating impact on health systems, critically ill patients, and fragile economies. As a result, Contract Medical Device Manufacturing has emerged as a strategic approach for companies aiming to enhance, or ensure, supply chain resiliency.
This article delves into the role contract manufacturing plays in diversifying the production and sourcing base for businesses, while also exploring how companies can strike a balance between cost-effectiveness and supply chain resiliency.
The Landscape of Medical Device Manufacturing
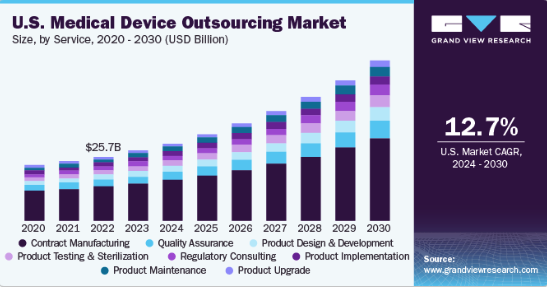
Image Credit: Grand View Research
To comprehend the important role of contract manufacturing in enabling supply chain resiliency, it is crucial to understand the dynamics of the medical device manufacturing landscape. Traditional manufacturing models often involve in-house production, leaving companies vulnerable to disruptions arising from geopolitical events, natural disasters, or global health crises. The COVID-19 pandemic, for instance, underscored the vulnerabilities of centralized production models as many companies faced challenges maintaining a steady supply of critical medical devices and components.
The global medical device outsourcing market size was estimated at $128.8 billion in 2023 and is anticipated to grow 12.8% per year from 2024 to 2030. To maintain control over product availability, OEMs must gain control of their supply chain.
The Benefits of Contract Medical Device
Manufacturing
Contract manufacturing in the medical device industry involves outsourcing the production of components or entire devices to specialized firms. This decentralized approach offers several advantages in terms of supply chain resiliency, including:
- Local Sourcing: relocating critical component sourcing and manufacturing processes back to domestic locations serves as a strategic buffer against disruptions in any single region. Through nearshoring and onshoring, companies can enhance the robustness and resilience of their supply chains, thereby mitigating risks associated with geopolitical uncertainties, natural disasters, or global disruptions. This approach not only fosters greater supply chain stability but also contributes to the overall economic sustainability and security of the onshoring country.
- Risk Mitigation: by strategically aligning with local contract manufacturing facilities, companies can mitigate risks associated with geopolitical, economic, or environmental factors, thereby reducing the impact of localized disruptions. For instance, a medical device company with production facilities in North America can navigate challenges arising from foreign crises, ensuring a continuous supply of products to meet global demand.
- Balancing Cost-Effectiveness: a key element of supply chain resiliency is the ability to maintain cost-effectiveness. Striking the right balance between these two aspects is a delicate task that requires careful consideration. Contract manufacturing allows companies to optimize costs through economies of scale achieved by leveraging the expertise and efficiency of specialized manufacturers.
- Agility in Response to Market Dynamics: medical device manufacturers often face fluctuations in demand and Contract manufacturing allows them to scale production up or down based on market needs. This flexibility is crucial in adapting to unforeseen circumstances, such as sudden spikes in demand during health crises or changes in regulatory requirements.
- Collaborative Partnerships: establishing strong partnerships ensures seamless communication, transparency, and the alignment of goals between the parties involved. This collaborative approach enhances the overall efficiency of the supply chain, contributing to both cost-effectiveness and resiliency.
- Technology and Innovation: advancements in automation, data analytics, and digital connectivity enhance the efficiency of manufacturing processes, reduce lead times, and improve overall supply chain visibility. By embracing innovation, companies can stay ahead of the curve and better navigate the complexities of the globalized medical device industry.
Image Credit: TarikVision/Shutterstock.com
In a recent article, experts forecasted that the COVID-19 pandemic was accelerating the digital transformation of supply chains. Prior to the pandemic, supply chains were undergoing a slowly evolving digital transformation. Disruptions caused by the pandemic, however, have significantly accelerated this transformation.
- Regulatory Compliance and Quality Assurance: ensuring regulatory compliance and maintaining high-quality standards are non-negotiable in the medical device sector. Contract manufacturing requires a comprehensive understanding of regulatory requirements in different regions. Contract manufacturers must adhere to stringent quality control measures to guarantee the safety and efficacy of medical devices. Companies that prioritize regulatory compliance and quality assurance can build a reputation for reliability and trustworthiness.
Conclusion
Contract Medical Device Manufacturing has emerged as a strategic imperative for companies seeking to enhance supply chain resiliency in the face of global uncertainties. The enhanced security offered by localized contract manufacturers acts as a robust risk mitigation strategy, allowing companies to navigate disruptions and ensure a steady supply of critical medical devices. Balancing cost-effectiveness with resiliency, fostering collaborative partnerships, embracing technological innovation, and prioritizing regulatory compliance are essential components of a successful contract manufacturing strategy. As the medical device industry continues to evolve, companies that invest in a resilient and agile supply chain through local contract manufacturers are better positioned to thrive in an unpredictable world.

